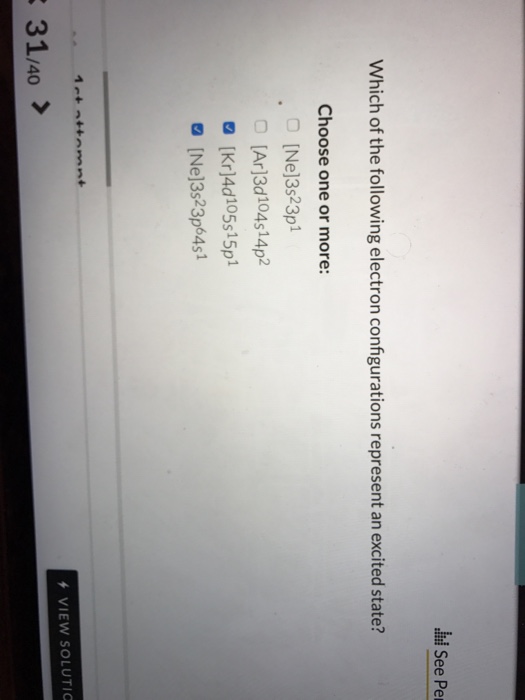
Which of the Following Electron Configurations Represent an Excited State
The ground-state electron configuration is Ne3s 2 3p 4 c. Look at the one before that.

Solved Which Of The Following Electron Configurations Of Chegg Com
The following electron configurations represent excited states.

. Ill be glad to check the others for you. As such Atomic electrons. Mg has atomic number of 12.
A 1 s2 2 s2 2 p4 3 s1 b Ar 4 s1 3 d10 4 p2 5 p1 mathbfcmathrmKr 5 s2 4 d2 5 p1. A This configuration is the ground-state electron configuration for a Ca atom. Which of the following statements is true concerning the electron configuration Ar4p2.
Which of the following electron configurations represents an excited state of the indicated atom. Which of the following electron configurations represents an excited state of the indicated atom. 1s2 2s2 2p2 3s1 Element Symbol.
See the answer. Which element is 23. Identify the element and write its ground-state condensed electron configuration.
There may be others there. The ground-state electron configuration contains three unpaired 6p. A neutral atom has the same number of protons as electrons.
As such the other three are excited. The following electron configurations represent excited states. Identify the element and write its ground-state condensed electron configuration.
A 1s2 2s2 3p2 4p1 b Ar 3d10 4s1 4p4 5s1 c Kr 4d65s25p1. 1s2 2s2 2p6 3s2 3p2 3s1 bNe. The following electron configurations represent excited states.
2 is He and that is the ground state. Details of Ground State vs Excited State Electron Configuration Example Practice Problems Explained. Which of the following electron configurations represents an excited state of the indicated atom.
An excited state of this element has the electron configuration Kr5s 2 4d 6 5p 2 6s 1. 1s2 2s2 2p6 B N. Up to 256 cash back Identify the following elements.
B This configuration cannot be the ground-state electron configuration for a Ca atom because it violates Hunds rule. Get Which Electron Configuration Represents An Excited State For A Potassium Atom MP3 For Free in Zai Airlinemeals uploaded by Conquer Chemistry. For the first one I count 18 2 3 23.
Lol See Periodic Table See Hint Which of the following electron configurations represent ground states and which represent excited states. Identify the element and write its ground-state condensed electron configuration. Scroll down to electron shell properties click on that and see that the ground state for Lu is Xe5d1 6s2 and that makes Xe6s2 4f1 an excited state.
Thats V isnt it. Group of answer choices aNa. One electron is transferred from 3s to 3p level to form excited state.
1s2 2s2 2p6 3s2 3p2 4s1 eHe. The other two options are settled atoms where the configuration is the correct one if you calculate it. 1s2 2s2 2p3 C P.
An electron is excited from the n1 ground state to the n3 state in a hydrogen atom. An Exited state means they filled some places sooner then they should. An excited state of the element has the electron configuration 1s 2 2s 2 2p 5 3s 1.
Ar4s13d104p25p1 Express your answer as a chemical symbol. 1s2 2s2 2p3 dP. It has 5s1 others but 5s2 is the ground state.
1s2 2s2 2p6 3s2 3p2 4s1. 1 s 2 2 s 2 2 p 6 3 s 1 3 p 1 represents an atom of magnesium in an excited state. Electron Configurations 4 items Drag and drop into the appropriate area below He2s 2p Ar3d1045-4p kr4d105525p Electronic State Ground State Excited State Ne3s 3p4s Drag and drop here.
Which of the following ground-state electron configurations corresponds to an atom that has the most negative value of the electron affinity. 1s2 2s2 2p6 3s2 3p2 4s1. Which of the following electron configurations I-IV represents an excited state.
The following electron configurations represent excited states. Identify the element and write its ground-state condensed electron configuration. Two elements that have the same ground-state valence shell configuration of ns2np2 are.
The ground state electronic configuration is 1 s 2 2 s 2 2 p 6 3 s 2. Ls2 2s2 3p2 4p1. Which of the following electron configurations represents an excited state of the indicated atom.
See the answer See the answer done loading. The which-electron-configuration-represents-an-excited-state-for-a-potassium-atom have 0438 and 73481. Identify the neutral element represented by this excited-state electron configuration then write the ground-state electron configuration for that element.
It must be excited since the ground state would be 1s2 to start. 1s2 2s2 2p6 cN. Which of the following electron configurations is impossible according to the Pauli exclusion principle.
Thus it will have 12 electrons.
Solved Which Of The Following Electron Configurations Chegg Com

Solved 1 Which Of The Following Electron Configurations Chegg Com

Oneclass Sort The Following Electron Configurations Of Neutral Atoms Based On Whether They Represent

Solved 1 Which Of The Following Electron Configurations Chegg Com
No comments for "Which of the Following Electron Configurations Represent an Excited State"
Post a Comment